Acute pain treatment requires safer medications... current solutions are beset by adverse effects
Opioids and NSAIDs are the two main drug classes used to treat acute pain. As is well known, opioids are driving a global public health crisis. Beyond addiction and abuse, even their ordinary medical use is associated with adverse gastrointestinal ("GI") effects, including nausea, vomiting and severe constipation. While NSAIDs are increasingly administered in conjunction with opioids, and can replace opioids in some settings, they are associated with significant GI ulcers and bleeding.
The Therapeutic Challenge: Today's NSAIDs
As features of human physiology, pain and inflammation are triggered by an increase in prostaglandin production in a body part that has sustained injury. Prostaglandins are regulated by cyclooxygenase (“COX”) enzymes, comprising two subtypes: COX-1 and COX-2; NSAIDs function by inhibiting COX enzymes. Prescribed for many types of acute pain, NSAIDs are routinely used for post-operative pain, alone or as part of multimodal analgesia.
The NSAID class includes aspirin and well-known consumer brands like Aleve (naproxen) and Advil (ibuprofen), as well as prescription-only medicines like celecoxib, ketoprofen and ketorolac. Because of their non-addictive nature and well-established efficacy, NSAIDs are central to the treatment of pain. However, as is widely known, NSAIDs can cause clinically significant GI ulcers and bleeding, a problem that has resisted solution since aspirin, the original NSAID, was introduced more than 100 years ago. As discovered by Dr. John L. Wallace and his colleagues, the root of this problem is that NSAIDs impair the body’s ability to maintain the mucosal layer that ordinarily protects the lining of the digestive organs. Several approaches have been attempted to address this problem, but all have proven unsatisfactory.
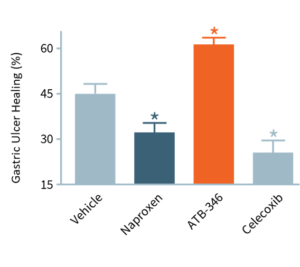
of gastric ulcers1
NSAIDs AND GASTRIC ULCERS
In this study, ulcers were induced in mice, which were then treated for four days. With no drug treatment (“Vehicle”), ulcers decreased in size by ~45%. Typically, NSAIDs (e.g., naproxen, celecoxib) significantly impair ulcer healing, whereas otenaproxesul (“ATB-346”) significantly accelerated ulcer healing. We believe this is the first instance of an NSAID demonstrating potential as an ulcer-healing agent. Otenaproxesul has also shown potential to improve bone healing.
COX-2 selective NSAIDs
While traditional NSAIDs block both COX-1 and COX-2 enzymes, the late 1990s saw the introduction of NSAIDs that specifically inhibit COX-2, on the premise that stomach damage was primarily due to the effects of suppressed COX-1. Although COX-2 selective NSAIDs were outstandingly successful commercially, the FDA eventually removed Vioxx and Bextra from the market, due to increased risk of cardiovascular events. Only celecoxib (branded as Celebrex) continues to be available in the US. To counteract cardiovascular concerns, it is often administered with low-dose aspirin, nullifying any gastroprotective advantage and intensifying damage to the small intestine.* 2,3,4
……………………….
* Damage to most of the small intestine was hidden from researchers until the availability of capsule endoscopy, a method of viewing the digestive tract via a swallowed capsule that contains a miniaturized camera. Traditional endoscopes are only able to inspect the stomach/duodenum or the large intestine.
……………………….
Enteric coatings
Proton pump inhibitors and H2 antagonists
Proton pump inhibitors (“PPIs”) and H2 antagonists (e.g., esomeprazole and ranitidine; branded as Nexium and Zantac) are often co-prescribed with NSAIDs to protect the stomach. However, this treatment strategy dramatically increases intestinal bleeding, ulcers, and risk of perforation.8 Notably, reduced stomach acidity can enable the survival of dangerous microbes, including C. difficile, with resulting opportunistic infections compounding the damage caused by the NSAIDs themselves.9
PROTON PUMP INHIBITORs: THE INTESTINAL RISKS
As depicted in the accompanying chart, intestinal damage in rats caused by naproxen (dark blue bars) and celecoxib (light blue bars) is worsened significantly when omeprazole (for GI protection) or low-dose aspirin (for cardiovascular protection) was co-administered. Omeprazole (“Omep”) is a common PPI.
Notably, when co-administered with a PPI, otenaproxesul (“ATB-346”) remained gastrointestinal-safe (although acid-reduction medication is unnecessary with otenaproxesul). Aspirin was also GI-safe when co-administered with otenaproxesul.
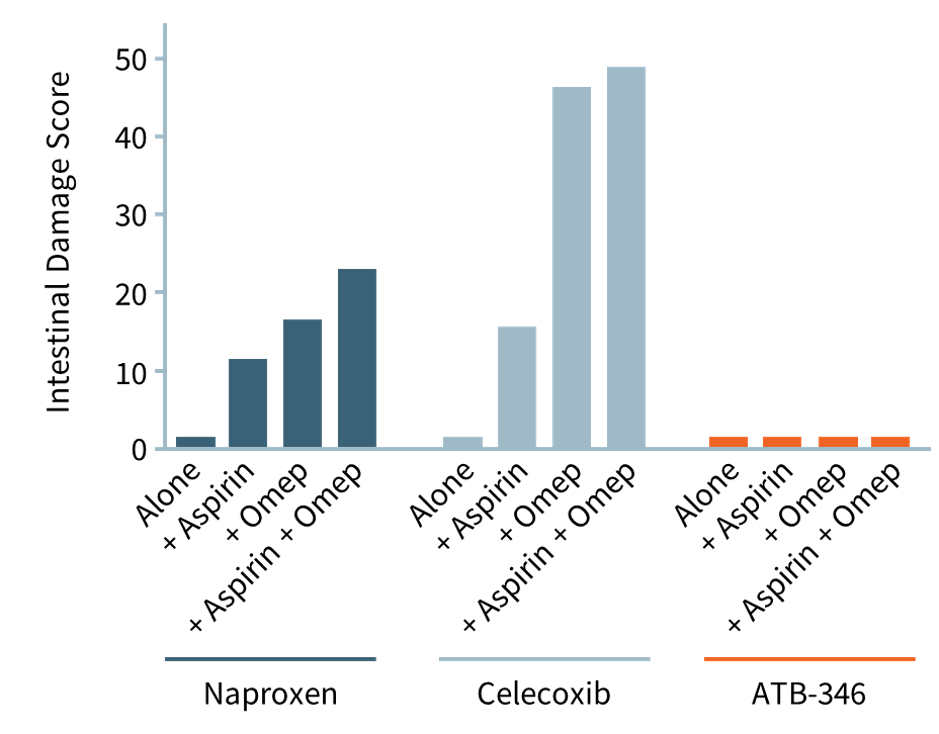
Unlike today's NSAIDs, otenaproxesul (ATB-346) is GI-safe, even when administered with aspirin10
Current Solutions Are Unsatisfactory...
Surgeons and other physicians rely on today’s NSAIDs to treat acute pain, both to replace opioids and as components of multimodal analgesia. However, NSAIDs can induce significant stomach and intestinal injury.
Hydrogen sulfide can help address this issue.
1. Wallace JL et al., Br J Pharmacol 2010;159(6):1236-1246.
2. Silverstein FE et al., JAMA 2000;284:1247–1255.
3. Laine L et al., Gastroenterology. 2003;127:395–402.
4. Wallace JL, Trends Pharmacol Sci. 1999;20:4–6.
5. Davies NM, J Pharm Pharm Sci 1999;2:5–14.
6. Endo H et al., Dig Liver Dis 2012;44:833–838.
7. Grosser T, Circulation. 2013;127:377–385.
8. Marlicz et al., Mayo Clinic Proceedings 2014;89(12):1699-1709.
9. Desilets AR et al., Consult Pharm 2012;27:114–120.
10. Blackler R et al., PLoS One 2012;7(4):e35196.
