We use cookies to ensure you get the best experience on our website. Learn more about our privacy policy.
Attention
This is an external link. Click “OK” to continue.
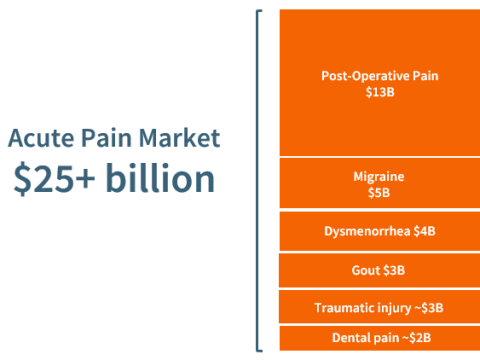
NSAIDs are increasingly employed in acute pain management to replace opioids, and as a component of multimodal analgesia, a growing practice whereby multiple drugs are administered to achieve optimal pain reduction in post-operative settings. While today’s NSAIDs are effective, they can cause a range of GI-related adverse effects. Even in short-term use, they triple the risk of serious GI outcomes.2 Celecoxib (branded as Celebrex) offers some improvement in GI safety, but its use is associated with cardiovascular toxicity. Beyond considerations of addiction and abuse, opioids can cause adverse GI effects, including nausea, vomiting and severe constipation—leading to patient discomfort and the cost of extended hospitalization.3
The drug remained GI-safe when administered to animals with compromised mucosal defense or pre-existing ulcers—situations in which today’s NSAIDs (including celecoxib) can cause ulcers and bleeding in humans. Otenaproxesul also remained safe when co-administered with aspirin. In addition, otenaproxesul did not elevate blood pressure when administered to rats with hypertension, in contrast to naproxen’s hypertensive effect.
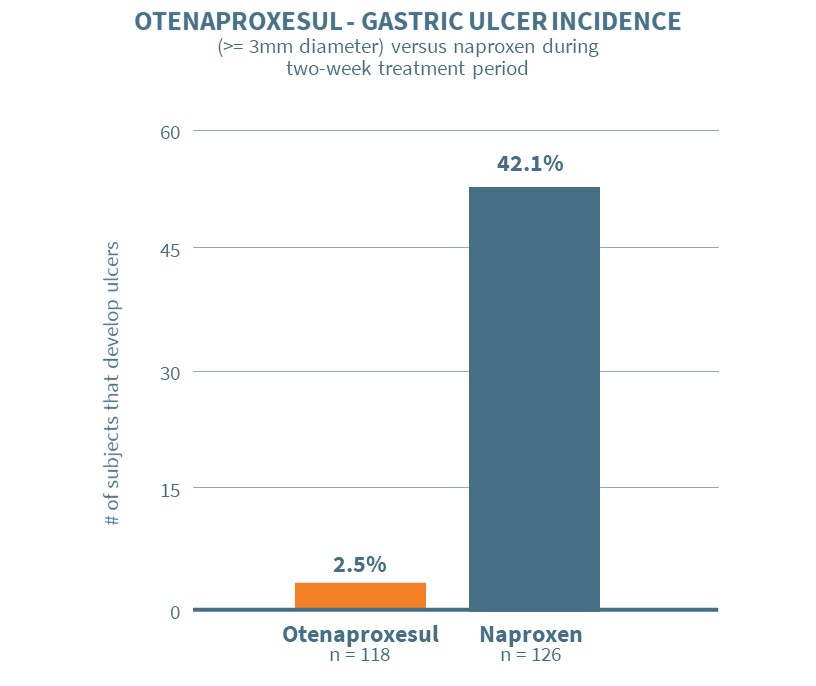
Otenaproxesul successfully met the primary endpoint in the study. Subjects on otenaproxesul exhibited an ulceration rate of 2.5% (3/118) versus an ulceration rate of 42.1% (53/126) for subjects on naproxen at the end of the treatment period, with a very high degree of statistical significance (p<0.0001).
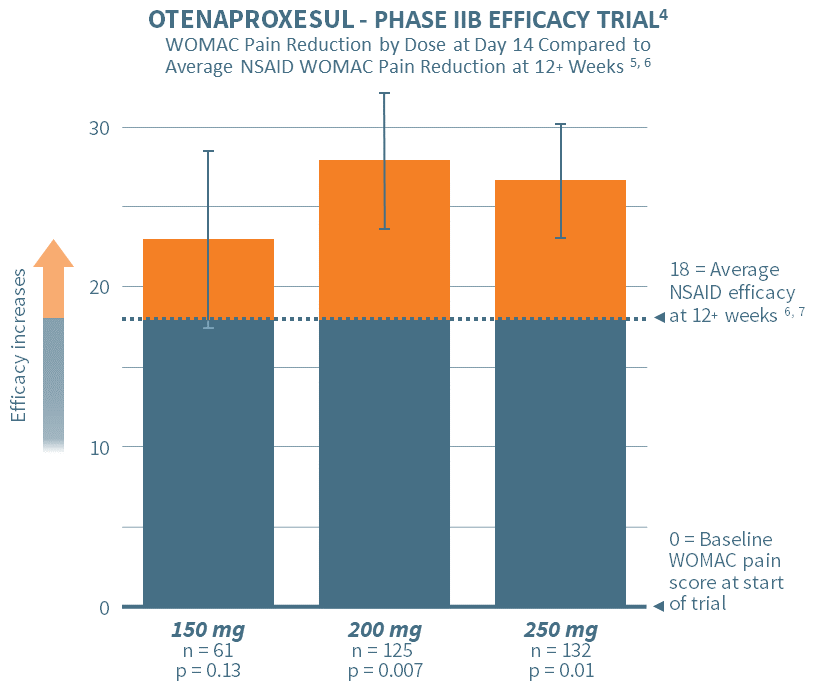
In June 2020, Antibe reported positive top-line results from its Phase IIB dose ranging, efficacy study designed to validate the efficacy of otenaproxesul in reducing chronic pain. The study involved 384 participants across 39 sites.
Subjects were randomized to placebo or one of three doses of otenaproxesul administered once daily: 150 mg, 200 mg or 250 mg. The primary endpoint was the change in the WOMAC pain subscale scores (the ‘gold standard’ in chronic pain assessment) over a 14-day treatment period. The results showed a high degree of statistical significance for the 200 mg and 250 mg doses, thereby demonstrating efficacy in pain reduction. The lowest dose, although not powered for statistical significance, showed a positive trend toward efficacy.
Further validating the WOMAC pain measures, otenaproxesul delivered profound COX inhibition, exceeding 90% for all doses, at very high degrees of statistical significance (p<0.0002).
The chart above depicts the pain efficacy of otenaproxesul in a 14-day treatment regimen for three doses. The vertical bars depict the improvement in WOMAC pain subscale scores for each of the doses after two weeks of administration. The horizontal dotted line indicates the average efficacy of NSAIDs after 12 (or more) weeks, as set out in Comparative Pain Reduction of Oral Non-steroidal Anti-inflammatory Drugs and Opioids for Knee Osteoarthritis: Systematic Analytic Review; Osteoarthritis and Cartilage, S.R. Smith et al., 2016.6
These results support the success of an earlier Phase IIA open-label, effectiveness study. Its primary endpoint was the clinical assessment of pain over the ten-day course of treatment at 250 mg once-daily (n = 12), delivering a significant WOMAC pain score reduction on day 10, at a very high level of statistical significance in comparison to baseline pain (p<0.001).
Since launching the clinical program for acute pain in early 2022, Antibe has transitioned to a faster-absorbing formulation. The new formulation’s benefits include: (i) rapid dissolution mechanics, accelerating otenaproxesul’s onset of action, a key benchmark for acute pain medications; and (ii) enhanced bioavailability, enabling a significant dose reduction compared to its previous formulation and a potential pathway to address chronic pain indications.
Antibe plans to conduct a single Phase II trial utilizing a surgical abdominoplasty model, recognized as one of the most reliable methods for evaluating analgesic efficacy in post-operative pain.
Post-operative pain serves as an ideal regulatory pathway to the broader acute pain market. With the lack of recent innovation in oral analgesics and the urgent need for non-addictive alternatives, Antibe expects otenaproxesul to be especially attractive to potential partners.
Further information about otenaproxesul’s commercial opportunity is available in our Corporate Presentation.
In light of the strong safety and PK data from the November 2023 clinical PK/PD study, Antibe has upgraded the design of the Phase II trial. Enhancements include the introduction of a placebo arm, a considerable increase in sample size, and the use of adaptive design methodology to manage statistical powering. Taken together, these enhancements may enable the Phase II trial to qualify as a pivotal trial for the US FDA. Trial initiation is set for calendar Q1 2024 with top-line results anticipated in calendar Q3 2024.
If the Phase II trial is successful, the Company will request an End of Phase II meeting with the U.S. FDA to discuss the Phase III program. If the Phase II trial is accepted as a pivotal trial, the Phase III program would involve a single trial using the hard tissue surgical model of bunionectomy. If the Phase II trial does not qualify as pivotal, the Company would conduct a Phase III abdominoplasty trial (replicating the Phase II trial) concurrently with the bunionectomy trial. Given the short treatment durations employed in acute pain trials, the Company expects to file otenaproxesul’s NDA with the U.S. FDA in 2026. Antibe intends to apply for marketing approval for a broad acute pain indication.
……………………………………
1. Allied Market Research, Biotech Advisors, DataBridge, DelveInsight, GlobalData, Statista, Transparency Market Research, Antibe internal estimates.
2. Fine M, Am J ManagCare (2013);19(16 suppl):S267-S272.
3. Whitman CJ et al., J Opioid Manag (Sep-Oct 2015)11(5):383-91.
4. Post hoc analysis. Results do not necessarily predict future efficacy results.
5. WOMAC pain subscale scores based on 500-point Likert scale; figures normalized to 100mm scale; shown with 95% confidence interval.
6. NSAID study data from Comparative Pain Reduction of Oral Non-steroidal Anti-inflammatory Drugs and Opioids for Knee Osteoarthritis: Systematic Analytic Review; Smith; S.R., Deshpande, B.R., Collins, J.E., Katz, J.N., Losina, E.; Osteoarthritis and Cartilage; 24: 962-972, 2016.
7. Adjusted mean change from baseline computed with assumption that subjects who withdrew early due to insufficient efficacy had zero (0) change from baseline WOMAC pain subscale score.
We use cookies to ensure you get the best experience on our website. Learn more about our privacy policy.
